Introduction: The safety and efficacy established by daratumumab (a CD38 monoclonal antibody)-based induction therapies for both transplant-eligible myeloma patients (based on CASSIOPEIA, and GRIFFIN trials) and transplant-ineligible patients (based on MAIA trial) have gained an accelerated momentum for adoption into regular clinical practice. In this context, to find the most optimal non-IMID based daratumumab-combination therapy as induction, we conducted a randomized phase 2 study to evaluate the safety and efficacy for in-class transition from bortezomib to ixazomib, stratified by transplant-eligibility and R-ISS.
Methods: Patients were randomized to receive Arm A [daratumumab, ixazomib and dexamethsone (DId) X 8 cycles)] or Arm B [(daratumumab, bortezomib and dexamethsone (DVd) x 3) followed by (DId x 5 cycles)] for the primary endpoint of ≥ very good partial response rate (VGPR) rate post-induction cycle 8. Several other secondary endpoints were evaluated including safety, overall repsonse rate (ORR), progression free survival (PFS) and overall survival (OS). Standard dosing schedule was used [DId: daratumumab IV 16 mg/kg on days 1, 8, 15, 22 every 28 days x 2 cycles; then on days 1, 15 every 28 days x 6 cycles and every 28 days during maintenance (or 1800 mg SC at the same schedule), ixazomib - 4 mg PO on Days 1, 8, and 15 every 28 days and dexamethasone - 40 mg PO on days 1, 8, 15 and 22 every 28 days; DVd: daratumumab IV 16 mg/kg on days 1, 8, 15, 22 every 28 days x 2 cycles; then on days 1, 15 every 28 days x 6 cycles and every 28 days during maintenance (or 1800 mg SC at the same schedule), bortezomib - 1.3 mg/m2 SC on days 1, 4, 8 and 11 every 21 day and dexamethasone - 40 mg PO on days 1, 8, 15 and 22 every 28 days]. Transplant eligible patients may receive stem cell collection and transplant after cycle 8. During the maintenance phase, patients receive DId as maintennace therapy for a total of 32 cycles. 52 subjects were enrolled at Winship Cancer Institute of Emory University between 07/2019 and 05/2022.
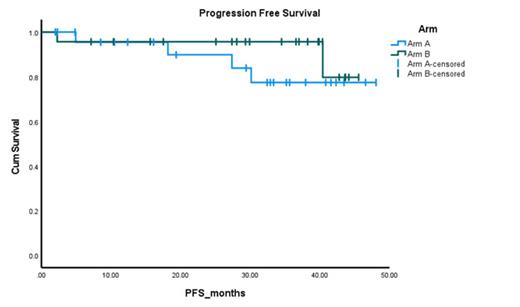
Results: The median age was 64 years for the 48 evaluable subjects [25 (52%) in arm A, 68 (48-87) and 23 (48%) in arm B, 61 (37-80), p<0.25]. 56% of the cohort was male (64 vs 48%, p=0.20), 48% were black (40% vs 56.5%, p=0.245). 20.8% had t(11;14), 29.2% had 1q abnormalities, 70.8% had hyperdiploidy, 25% had complex karyotype and 10.4% were high-risk [defined as presence of del17p, t(4;14) or t(14;16)]. 38 (81%) patients underwent an autologous stem cell transplant (72% in Arm A and 90.9% in Arm B, p=1.00). The primary endpoint favored arm B (Arm A vs Arm B ≥VGPR rate: 28% vs 56.5%, p<0.043). Similarly, the ORR favored arm B as well (Arm A vs Arm B ORR: 76% vs 95.7%, p<0.062). Post transplant ≥VGPR rates and ORR were 88.9% vs 95%, p=0.458 and 100% vs 100%, p=NS, respectively. At a median follow up of 35.2 months, 80% of the patients remain progression free as shown in figure 1. The adverse event profile was similar across both cohorts (grade 3/4 events occurred in 6 (24%) vs 7 (30%) for Arm A and B, respectively, p=0.27). There was no grade 3/4 peripheral neuropathy (PN), though grade 1/2 PN were higher in Arm B. There were two secondary primary malignancy reported in Arm B (prostate adenocarcinoma, esophageal adenocarcinoma). All together the safety signals were comparable between both arms.
Conclusion: This is one of the first studies showing the safety and efficacy of non-IMID based dara-combination induction therapies with in-class transition of bortezomib to ixazomib yielding higher response rates post-induction and post-transplant without compromising on the safety. 3-year PFS rate of close to 80% across both arms is for daratumumab and proteasome inhibitor combination therapy are highly encouraging and may be an alternative regimen for patients that may not tolerate IMIDs or that have a contraindication to receive IMIDs.
Disclosures
Nooka:Aduro Biotech, Amgen, Arch Oncology, Bristol Myers Squibb, Cellectis, Genentech, GlaxoSmithKline, Janssen, Karyopharm, Kite Pharma, Merck, Pfizer, Takeda: Honoraria, Research Funding; Adaptive Biotechnologies, Amgen, BeyondSpring, Bristol Myers Squibb, Cellectar Biosciences, GlaxoSmithKline, Janssen, Karyopharm, Oncopeptides, ONK therapeutics, Pfizer, Sanofi, Secura Bio, Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Joseph:Janssen Oncology: Consultancy; BMS: Honoraria. Hofmeister:Janssen: Membership on an entity's Board of Directors or advisory committees; Pfizer: Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Sanofi: Research Funding. Dhodapkar:Sanofi: Membership on an entity's Board of Directors or advisory committees; Lava Therapeutics: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees. Cortoos:Janssen: Current Employment, Current equity holder in publicly-traded company. Lin:Janssen Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Labotka:Takeda Pharmaceutical Company Limited.: Current Employment. Noga:Takeda Oncology: Current Employment, Current equity holder in publicly-traded company. Kaufman:Abbvie: Consultancy; Incyte: Consultancy; BMS: Consultancy; Sanofi: Consultancy. Lonial:Novartis: Research Funding; AbbVie Inc, Amgen Inc, Bristol-Myers Squibb Company, Celgene Corporation, Genentech, a member of the Roche Group, GlaxoSmithKline, Janssen Biotech Inc, Novartis, Pfizer Inc, Takeda Pharmaceuticals USA Inc: Consultancy, Other: Advisory Committee; TG Therapeutics Inc: Other: Board of Directors with Stock; Bristol-Myers Squibb Company, Janssen Biotech Inc, Novartis, Takeda Pharmaceuticals USA Inc.: Other: Contracted Research, Research Funding; Janssen: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal